
That the lowest Carr’s Index was associated with the highest variability of fill weight. Previously been identified as key properties for a powder for encapsulation, using both dosing disc andĭosator type machines (H&K GKF400 and Zanasi LZ64 respectively).įor different grades of MCC it was found that 101 grades with Carr’s Index of 37% or 102 grades withĬarr’s Index of 27% gave the least variability of fill weights on both types of filling machine.įor blends of ascorbic acid and MCC, with Carr’s Index values ranging from 12% to 21% it was found TheĪssessment is based on two key physical properties (Carr’s Index and plug strength) which have The purpose of the work in this paper was toĪssess the most suitable types of lactose for use in simple dry blended capsule formulations. Lactose is available in a wide number of types (milled & sieved with different particle sizes, anhydrous,Īgglomerated and spray dried) for various applications. % Relative Humidity 80 100 Lactose is water soluble and does not contribute to the blockage of some sinkers used inĬapsule dissolution testing. % Weight change 2.0 SuperTab® 30GR Sorption SuperTab® 30GR Desorption SuperTab® 21AN Sorption SuperTab® 21AN Desorption 1.5 The hysteresis in theĪnhydrous lactose plot is attributable to crystallisation of the anhydrous αlactose component to Water at 90% RH, and anhydrous lactose absorbs approximately 1%. Lactose monohydrate absorbs only about 0.2% ND Lactose monohydrate and anhydrous lactose both exhibit very low hygroscopicity (see theĭynamic vapour sorption (DVS) plots in figure 2). Table 1 shows the measured formaldehyde and acetaldehyde content of a range of capsule diluents Use of lactose in hard gelatin capsules (2). AnĮxtensive review of the affect of aldehydes on gelatin capsules can be found in reference 1. That cause insolubility of hard gelatin capsule shells resulting in extended dissolution times. Aldehydes have been implicated in the cross linking reactions Phosphate There are several beneficial properties of lactose that make it suitable in hard gelatin capsuleįormulations. Frequency of citation in the FGA IID 50%Ġ% Lactose MCC Starches Mannitol Dicalcium Figure 1 shows the frequency of use of 5 commonĭiluents in hard gelatin capsule formulations based on analysis of the FDA Inactive Ingredients Database Lactose is a common diluent in hard gelatin capsules. Printing, and they are patient friendly being easy to swallow and providing taste masking for unpleasant

#Lactose supertab 11sd trial
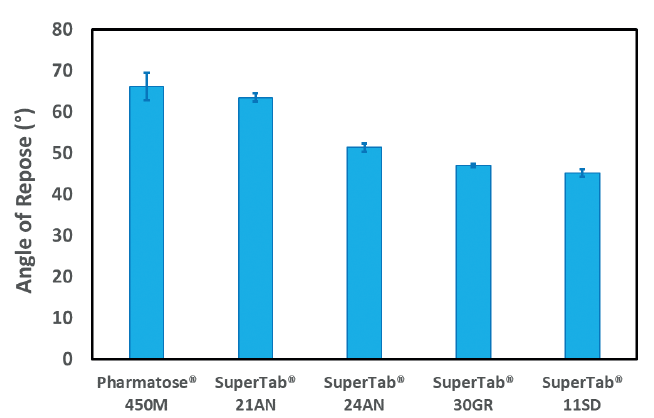
Tablets they are easy to formulate and manufacture, formulations can be developed with minimalĪmounts of active pharmaceutical ingredient (API) and they are easily blinded for clinical trial purposes.Īdditionally they provide a ready means of identification through the choice of capsule shell colour and Hard gelatin capsules are a common oral solid dosage for immediate release formulations. Strongest plugs of the grades of lactose monohydrate. SuperTab® 21AN gave the strongest plugs of all grades of lactose tested, and SuperTab® 30GR gave the In hard gelatin capsules based on their flowability (Carr’s Index). Superdisintegrants ® ® ® SuperTab 21AN, SuperTab 30GR and sieved grades of Pharmatose are all suitable for encapsulation Unformatted text preview: Use of lactose in hard


 0 kommentar(er)
0 kommentar(er)
